AN-F03 Studies of fermentation and processes to optimize the effectiveness of starter cultures

Instruments to which this note applies: Biocal 2000, Biocal 4000
Target use: Research and Quality Control related to fermentation of foods including dairy products, beer, wine and spirits and other fermentation processes.
Introduction
발효는 선사시대부터 보존이나 맛을 위해 탄수화물이 풍부한 고형 식품이나 액체를 처리하는데 사용되었습니다. 발효는 등온 전도 열량계로 연속적으로 측정하기 쉬운 유기산, 알코올 및 가스의 조합물을 생성할 수 있습니다.
발효의 효과는 균주의 선택, 농도, 온도 등과 같은 요인에 의해 결정됩니다. 이러한 요인들에 의한 발효반응의 효과는 등온열량 곡선에서 매우 잘보이고 외부 요인없이 모니터링 할 수 있습니다. 결과는 버튼클릭으로 검색할 수 있습니다. 이것은 등온열량측정법이 다른 식품에 발효가 어떻게 진행되는지 평가할 수 있는 편리하고 효과적인 도구가 되며, 단순히 등온열량측정 곡선을 비교하여 온도, 스타터 농도 등의 함수로 발효 속도를 쉽고 빠르게 비교할수 있습니다. 125ml 샘플 바이알를 사용하는 Calmetrix Biocal과 같이 큰 샘플 세포 열량계를 이용한다면 채소, 육류 또는 치즈 그리고 다른 고형 그리고 액체 식품과 같이 연구할 수 있는 식품의 범위가 늘어날 것입니다.
이 Application Note는 23 °C에서 2종류의 다른 스타터 배양으로 처라한 저온살균 우유샘플에서의 열량변화를 보여준다.
Test Protocol
A small amount of starter culture (the fermented product) was added to milk, mixed and loaded into 125 ml sample vials that were placed in the calorimeter. The two samples only differed in the concentration of starter culture that was used (0.2% and 1% by mass of fermented product.
Results and Interpretation
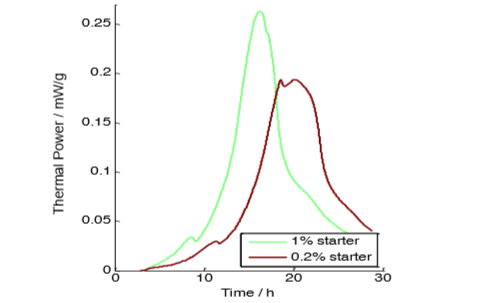
Both samples show the typical behavior during milk fermentation. First one exponential phase that ends with a sudden drop in activity, then the second main phase. The sample with lower starter concentration is slower.
The sample with less culture lags about 3 h behind the other sample as the microorganisms with lower initial concentration take longer time to multiply to reach a certain number of bacteria (and a certain thermal power). As the ratio between the initial concentrations was 5 and it took 3 h for the lower concentration to reach the level of the higher one, the time constant of the exponential growth can be inferred easily from solving the equations for exponential growth:
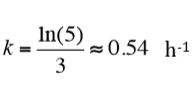
This corresponds to a doubling time of about 1.3 h, i.e., the bacteria divide every 1.3 h.
Conclusion
Isothermal calorimeters such as Calmetrix’s Biocal models are an effective and easy-to-use tool for the study of fermentation processes, especially such processes for which it is difficult to follow the reaction with pH-sensors, like non-acidifying cultures or solid foodstuffs. One example of such applications is to study the effectiveness of starter cultures, as shown in this Note. After only a few hours, or even earlier at higher concentration, the activity of the starter culture can be measured effectively through a simple visual interpretation of the calorimetry graph. A closely related use may also be to check the content of active microorganisms in pro-biotic foods.
References
- Stulova, I., et al., Fermentation of reconstituted milk by Streptococcus*thermophilus: Effect of irradiation on skim milk powder. Int. Dairy J., 31 (2013) 139-149
- Wadsö, L. and F. Gómez Galindo, "Isothermal calorimetry for biological applications in food science and technology." Food Control 20(10) (2009) 956-961.
- Riva, M., et al., Growth and fermentation activity of Streptococcus* thermophilus and Lactobacillus* bulgaricus in milk: a calorimetric investigation. Annali di Microbiologia ed Enzimologia, 47 (1997) 199-211 (in English).
