Introdution
Measurement of a known reaction enthalpy is useful tool to assess the performance of any instrument. Here, a standard calorimetry test is performed using the aqueous reaction of sodium hydroxide and nitric acid according to the following reaction scheme. The standard enthalpy of neutralization for hydrogen ions by hydroxyl ions strong acids/bases is -57.3kJ mol-1. This experiment will be used to demonstrate that the Micro Reaction Calorimeter(μRCTM) from THT can be used to accurately measure the enthalpy of this and similar processes, and that it can determine titration end point.
Experimental
In this experiment the sample vial was made up with 1 mL of a 0.05M sodium hydroxide solution and a standard magnetic stirrer. This vial was then placed in the sample holder in the calorimeter. A 100μL syringe of 1M nitric acid solution was fitted to the instrument. The experiment was set up to perform twenty 5 μL injection of the acid into the base and to monitor the heat evolved from this process. It was expected that a full neutralization of the base should occur after 10 injections (50 μL).
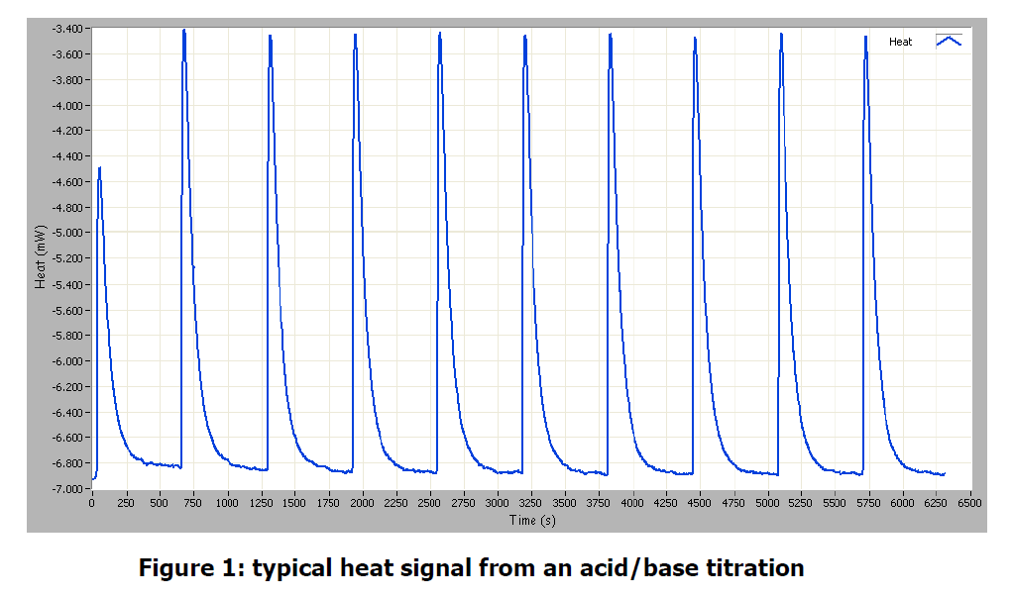
Result
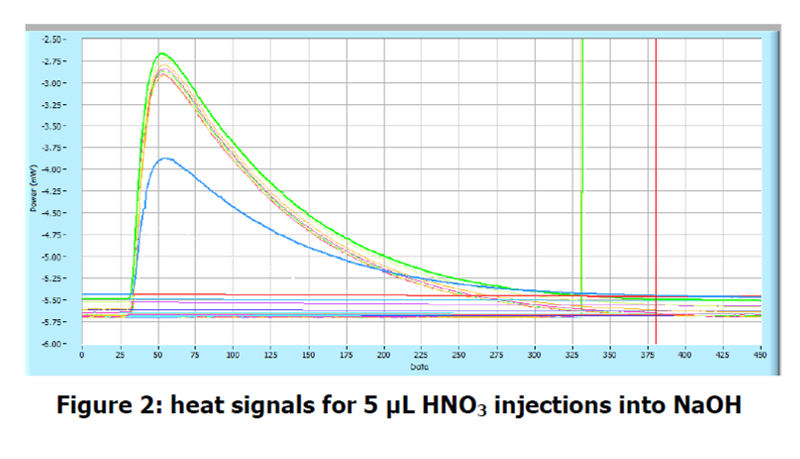
Each injection gives a large peak in the heat signal of the instrument. An increase in heat output corresponds to an exothermic process occurring in the vial. It is important to not that first injection in a titration experiment typically gives a smaller heat signal than subsequent injection. It has been suggested that this is caused by the pressure change when piercing the septum pushing some of the sample into the syringe, although the phenomenon has not been fully explained. The figure below shows the results from the injections overlayed in the analysis software. A table of integrated peaks is also shown to illustrate the reproducibility of the initial peaks and the subsequent end-point of the titration. These results suggest the end point occurs at around peak 10, which corresponds to an equimolar solution of acid and base. The enthalpy of reaction for this process can be seen to be -56.6 kJ mol-1 may be due to solution in the vial, although it is within the typical error margin of the instrument (± 2%).
Discussion and Conclusions
These data show the excellent consistency of the μRCTM for collection of reaction enthalpy data. The enthalpy of neutralization has been determined with good accuracy, and the drop in heat output occurs at the expected titre base on the number of injections.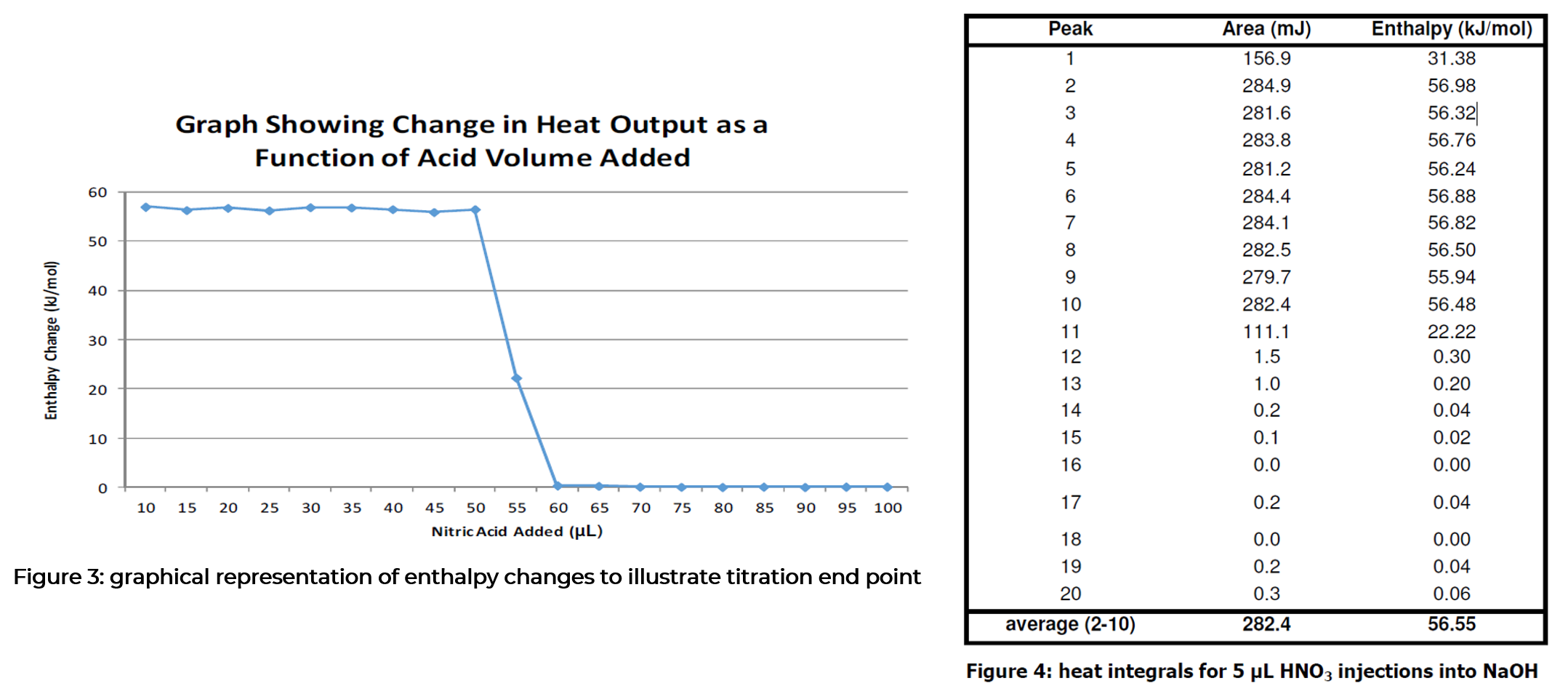
The THT Micro Reaction Calorimeter (µRC™) has a wide range of applications in the chemical, pharmaceutical and related fields. This brochure shows how using a microcalorimeter can help chemists and engineers in everyday tasks.
Some of the applications detailed within this brochure include:
- Reaction Kinetics
- Process Development
- Scanning Calorimetry
- Thermal Stability
- Heat Capacity Measurement
- Adhesive Curing Reactions
- Waste Management
- End Point Determination
- Hazard Analysis
All chemical, physical and biological reactions are accompanied by heat change. These reactions, though sometimes subtle, can be measured using calorimetry. This powerful analysis method works without modification of the sample or process. The THT Micro Reaction Calorimeter is based on power compensation technology making it faster in both signal response and temperature variation. Designed for maximum flexibility, the µRC has the capability to measure both kinetic and thermodynamic parameters in both rapid and slow reactions and in solids, liquids or gases. Measurements made by calorimetry are non-destructive and non-invasive making it valuable for initial analyses and for systems where other techniques fail. With the µRC there is minimal sample preparation and no limitation on the physical state of the material. Systems can be studied under ambient or modified environmental conditions.
Specification
- Baseline Noise: From 5μW
- Dynamic Range: 5μW to 600mW
- Temperature Range: -5°C to 170°C
- Standard Modes of Operation: Isothermal/Titration/ Scanning
- Optional Modes: Pressure - pressurise cell up to 10bar
End Point Determination of Acid Base Titration by μRC - Scanning Rate: Up to 2°C/min
- Isothermal Stability: +/- 0.0001°C over extended time period
- Cell Volume: 1.5 ml
- Cell Type: Removable glass vial
- Injection Volume: 1 to 250 μl
- Temperature Control: Peltier based (no external cooling)
- Stirring Speed: 0 - 400 rpm
- Measurement Principle: Power compensation
- Connection to PC: via USB cable
- Footprint (width x depth x height): 19 x 31 x 35 cm
- Certain other specifications may be possible by discussion
하기
