Introduction
In addition to Titration mode, the THT μRC Micro Reaction Calorimeter has facility to operate as a differential scanning calorimeter. As a preliminary example in this area the thermal denaturation of the ‘model’ protein Lysozyme has been studied.
Lysozyme occurs in tears, other exocrine secretions, and in very large amounts in chicken egg albumen. Its antibacterial action depends on the cleavage of the β (1→4) glycosidic linkage between alternating units of Nacetylmuramic acid and N-acetylglucosamine that form long-chain mucopolysaccharides in bacterial cell walls.
Experimental
4mg/ml of lysozyme from chicken egg white (Sigma L-6876) was prepared in 20mM Sodium Acetate buffer, pH
5.2. 1.7ml of lysozyme was added to the removable HPLC style glass vial sample holder and loaded into the μRC. A second vial was filled with buffer and used in the reference cell. The system was ramped at scan rate of 1 ℃/min.
Results
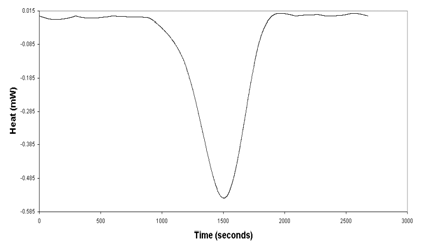
RAW DATA - BASELINE CORRECTED
Heating Scan 1 ℃/min Lysozyme 4mg/ml in 20mM
Na Acetate Buffer, pH 5.2
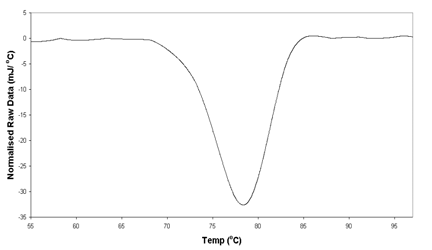
NORMALISED RAW DATA
Heating Scan 1 ℃/min Lysozyme 4mg/ml in 20mM
Na Acetate Buffer, pH 5.2
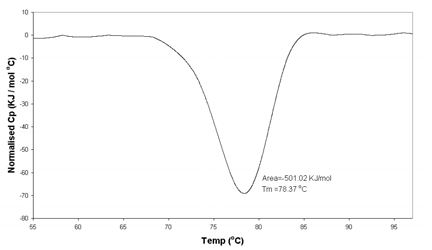
NORMALISED Cp
Heating Scan 1 ℃/min Lysozyme 4mg/ml in 20mM
Na Acetate Buffer, pH 5.2
The μRC yields results for lysozyme of Tm = 78.4 ℃ and ΔH = 501 KJ/mol
Discussion and Conclusions
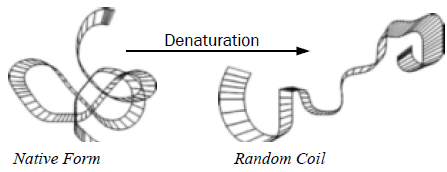
During heating the lysozyme undergoes a conformational change resulting in an endothermic transition. This is clearly observed in the μRC. Peptide bonds generally remain intact and the protein retains its original primary structure. Lysozyme is one of the few natural proteins that exhibits thermal reversibility. Upon cooling the denatured protein can return to its native state and resume its specific biological activity.
Reference
Thermal Hazard Technology Micro Reaction Calorimeter - μRC™ Technical Application Note 14, Scanning - Protein Denaturation
The THT Micro Reaction Calorimeter (µRC™) has a wide range of applications in the chemical, pharmaceutical and related fields. This brochure shows how using a microcalorimeter can help chemists and engineers in everyday tasks.
Some of the applications detailed within this brochure include:
- Reaction Kinetics
- Process Development
- Scanning Calorimetry
- Thermal Stability
- Heat Capacity Measurement
- Adhesive Curing Reactions
- Waste Management
- End Point Determination
- Hazard Analysis
All chemical, physical and biological reactions are accompanied by heat change. These reactions, though sometimes subtle, can be measured using calorimetry. This powerful analysis method works without modification of the sample or process. The THT Micro Reaction Calorimeter is based on power compensation technology making it faster in both signal response and temperature variation. Designed for maximum flexibility, the µRC has the capability to measure both kinetic and thermodynamic parameters in both rapid and slow reactions and in solids, liquids or gases. Measurements made by calorimetry are non-destructive and non-invasive making it valuable for initial analyses and for systems where other techniques fail. With the µRC there is minimal sample preparation and no limitation on the physical state of the material. Systems can be studied under ambient or modified environmental conditions.
Specification
- Baseline Noise: From 5μW
- Dynamic Range: 5μW to 600mW
- Temperature Range: -5°C to 170°C
- Standard Modes of Operation: Isothermal/Titration/ Scanning
- Optional Modes: Pressure - pressurise cell up to 10bar
uRC에 의한 단백질 변성 (Protein Denaturation) 측정 분석 - Scanning Rate: Up to 2°C/min
- Isothermal Stability: +/- 0.0001°C over extended time period
- Cell Volume: 1.5 ml
- Cell Type: Removable glass vial
- Injection Volume: 1 to 250 μl
- Temperature Control: Peltier based (no external cooling)
- Stirring Speed: 0 - 400 rpm
- Measurement Principle: Power compensation
- Connection to PC: via USB cable
- Footprint (width x depth x height): 19 x 31 x 35 cm
- Certain other specifications may be possible by discussion
하기
