Kinetics Determination
Kinetic analysis can be tedious and time consuming.
The use of micro calorimetry to obtain kinetic parameters has been recognized internationally and is an essential tool in kinetics research.
Since reaction rate is directly proportional to heat-flow, it is possible to utilise the data from a μRC calorimeter to calculate reaction conversion at any point.
From here, every data point within the experiment can be used for an “initial-rate” type determination. Fitting of models to the calorimetric data can also be used to elucidate detailed kinetic mechanism.
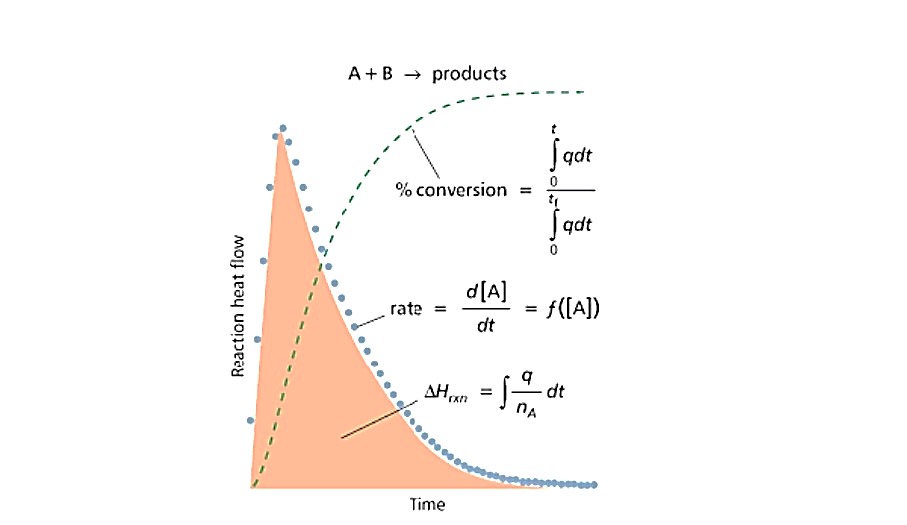
For slower reactions the Micro Reaction Calorimeter can be used as an isothermal (or step-isothermal) calorimeter giving rapid access to traditional kinetic methods.
A kinetics fitting and modelling package (μRC-Pro) is available as an option with the Micro Reaction Calorimeter. This software is specifically written for use with the THT μRC and contains automatic data importing, advanced data correction, kinetic fitting and modelling. Complex models may be used to provide fitting to consecutive, non-integral, parallel and auto-catalytic kinetics with highly efficient data optimisation routines.
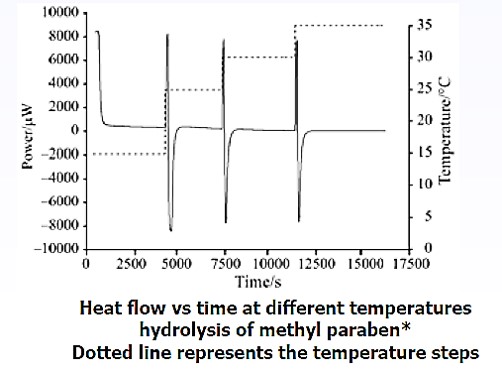
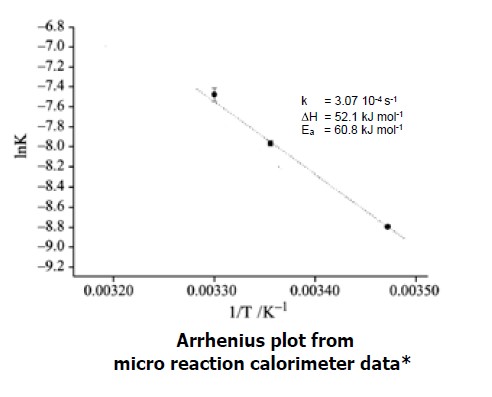
* reference: M. A. A. O’Neill et al : Journal of Thermal Analysis and Calorimetry, Vol. 84 (2006) 2, 301–306
The THT Micro Reaction Calorimeter (µRC™) has a wide range of applications in the chemical, pharmaceutical and related fields. This brochure shows how using a microcalorimeter can help chemists and engineers in everyday tasks.
Some of the applications detailed within this brochure include:
- Reaction Kinetics
- Process Development
- Scanning Calorimetry
- Thermal Stability
- Heat Capacity Measurement
- Adhesive Curing Reactions
- Waste Management
- End Point Determination
- Hazard Analysis
All chemical, physical and biological reactions are accompanied by heat change. These reactions, though sometimes subtle, can be measured using calorimetry. This powerful analysis method works without modification of the sample or process. The THT Micro Reaction Calorimeter is based on power compensation technology making it faster in both signal response and temperature variation. Designed for maximum flexibility, the µRC has the capability to measure both kinetic and thermodynamic parameters in both rapid and slow reactions and in solids, liquids or gases. Measurements made by calorimetry are non-destructive and non-invasive making it valuable for initial analyses and for systems where other techniques fail. With the µRC there is minimal sample preparation and no limitation on the physical state of the material. Systems can be studied under ambient or modified environmental conditions.
Specification
- Baseline Noise: From 5μW
- Dynamic Range: 5μW to 600mW
- Temperature Range: -5°C to 170°C
- Standard Modes of Operation: Isothermal/Titration/ Scanning
- Optional Modes: Pressure - pressurise cell up to 10bar
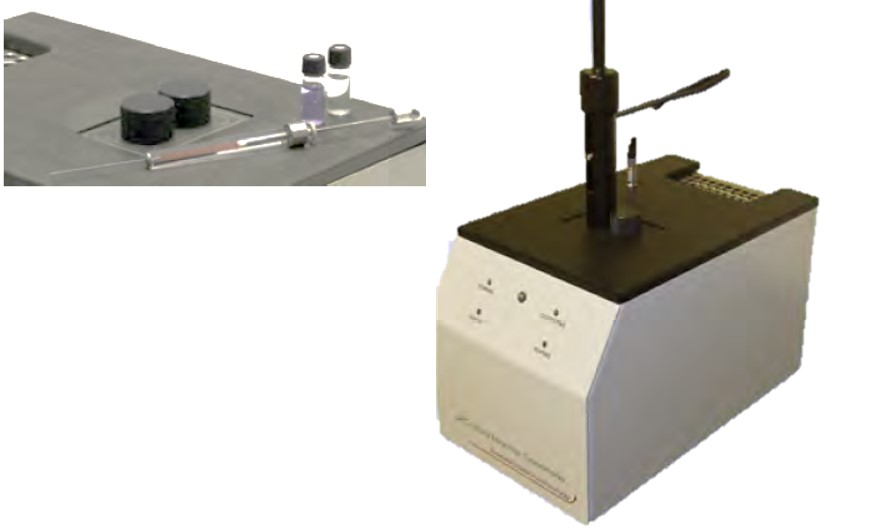
μRC에 의한 반응속도 측정 (Kinetic Determination) - Scanning Rate: Up to 2°C/min
- Isothermal Stability: +/- 0.0001°C over extended time period
- Cell Volume: 1.5 ml
- Cell Type: Removable glass vial
- Injection Volume: 1 to 250 μl
- Temperature Control: Peltier based (no external cooling)
- Stirring Speed: 0 - 400 rpm
- Measurement Principle: Power compensation
- Connection to PC: via USB cable
- Footprint (width x depth x height): 19 x 31 x 35 cm
- Certain other specifications may be possible by discussion
하기
