
Instruments to which this note applies : Biocal 2000, Biocal 4000
Target use : Research and Quality Control in lactic acid fermentation or pickling of vegetables.
Introduction
젖산 발효는 요구르트와 같은 발효 유제품에서 발생하는 것으로 가장 일반적으로 알려져있지만, 채소 보존에서도 사용이 됩니다 [1]. 후자의 가장 일반적인 예는 아마도 양배추와 무를 포함한 다른 종류의 채소로 만들어진 한국의 김치와 “sauerkraut”라고 불리는 독일, 폴란드 그리고 다른 동유럽 국가에서 먹는 발효 양배추일 것입니다. 당근 또한 이러한 과정으로 보존이 됩니다[2]. 이러한 제품들을 만들기 위해서 채소를 손질된 채소는 소금에 절이고 진공상태인 용기에 옮깁니다. 식염수로 채소에 있는 젖산균을 번식하고 많은 다른 미생물이 견딜수 없을 정도의 pH까지 낮아집니다. 젖산균은 또한 곰팡이와 같은 호기성 유기체의 번식을 효과적으로 막기위해서 산소를 소비합니다.
등온열량계는 단순히 등온 열량 측정 곡선을 비교하여 젖사발효와 산세 공정의 효율 차이를 평가할수 있는 편리하고 비용이 효율적인 도구입니다. Biocal과 같이 125ml의 폴리프로필렌 또는 스테인레스스틸 엠풀로된 큰 샘플 셀을 사용하는 열량계를 사용하면 과일과 채소 혹은 다른 모양과 크기로 잘린 조각들로 잘린 과일과 채소의 연구 적용법위가 늘어날 것입니다.
이 Application Note는 마늘을 첨가한 당근과 첨가하지 않은 당근의 젖산 발효로 발생하는 열 변화를 보여줍니다.
Test Protocol
Peeled and grated carrots were mixed with 1% sodium chloride, and loaded into a Calmetrix Biocal isothermal calorimeter at 20 °C. The samples of about 100 g were placed in standard polypropylene wials, to which a 15 cm stainless steel tube (inner diamter 0.6 mm) has been attached as an outlet for the produced cabon dioxide. A steel mass in a polyethylene bag was used to keep the carrots down in the liquid. Two grades of grating (3 and 1 mm) and the replacement of 1/3 of the carrots with garlic were tested.
Results and Interpretation
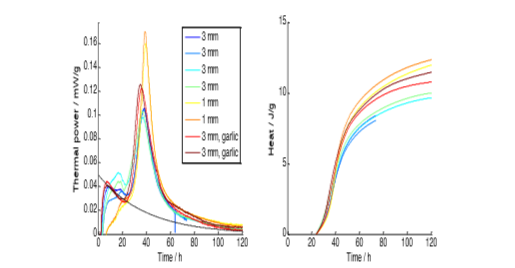
3.5 and the products had the typical flavour of non-
matured lactic acid feermented vegetables.
The thermal powers had a typical microbial peak starting after about 40 h, but before this a substantial part of the heat probably came from aerobic and anaerobic processes in the wounded carrot tissue.
The heat produced during the primary fermentation was about 10 J/g. The thermal power decreased to low non-‐zero values after the main peak. Fine grating gave a sharper peak, but the replacement of a large fraction of the carrots with garlic did not make any difference.
The enthalpy of the conversion of glucose to lactic acid and carbon dioxide is about ‐100 kJ/mol [3], so about 1/3 of the sugars (carrots typically contain 6% fructose and glucose) were consumed and about 0.01 g of lactic acid was produced per gram carrot.
Conclusion
Isothermal calorimetry is a powerful and simple to use technique to study different types of fermentation processes in the food industry [4], including the fermentation of vegetables, but also other types of food items, e.g. rye bread fermentation [5] or probiotic foods and cheese [6;7;8].
References
-
Steinkraus, K.H., Lactic acid fermentation in the production of foods from vegetables, cereals and legumes. Antonie van Leeuwenhoek, 49 (1983) 337 - 348.
-
Niketic-Aleksic, G.K., M.C. Bourne, and J.R. Stamer, Preservation of carrots by lactic acid fermentation. J. Food Sci., 38 (1973) 84 - 86.
-
Forrest, W.W., D.J. Walker, and M.F. Hopgood, Enthalpy changes assiciated with the lactic fermentation of glucose. J. Bacteriol., 82 (1961) 685 - 690.
-
Wadsö L, Gómez Galindo F. Isothermal calorimetry for biological applications in food science and technology. Food Control 2009; 20(10): 956 - 961.
-
Mihhalevski A, Sarand I, Viiard E, Salumets A, Paalme T. Growth characterization of individual rye sourdough bacteria by isothermal microcalorimetry. J Appl Microbiol 2010; 110: 529 - 540.
-
Schäffer B, Keller B, Daróczi L, Lorinczy D. Examinatio of growth of probiotic microbes by an isoperibolic calorimetry. J Thermal Anal Calorim 2010; 102: 9 - 12.
-
Schäffer B, Szakály S, Lorinczy D. Examination of the growth of probiotic culture combinations by the isoperibolic batch calorimetry. Thermochim Acta 2004; 415: 123 - 126.
-
Szily B, Óbert G, Schäffer B. Detection of probiotic microbes in probiotic cheese spreads by isothermal microcalorimetry. J Thermal Anal Calorim 2005; 82: 245 - 247.
