Introduction
The Accelerating Rate Calorimeter has never been promoted for vent size applications because of the small sample size and the general use of this instrument for a very wide range of sample types. Adiabatic calorimeters designed to measure vent sizing typically take larger samples and have an aim for a low phi value. However in their design the adiabaticity is not very high. Vent size instruments, usually have a system for pressure equalization. This is the aim of keeping the pressure around the container which holds the sample the same as that within the container. To do this, gas must be added to the calorimeter. This is often added without heating the gas or with heating to close to the sample temperature. Such an approach can cause significant problems, although the heat capacity of the gas is rather low, its thermal transfer is very slow, therefore it takes time to heat to the temperature of the sample. This must give an error in the test. However doing this type of pressure equalization means that a low phi, low mass sample container can be used.
Vent Sizing with THT ARC
The Thermal Hazard Technology Accelerating Rate Calorimeter has been designed such that an option is available to allow measurement on the type of reactive systems for which vent sizes may be required. This option combines with the high volume low φ test cells. The vent size option with the THT Accelerating Rate Calorimeter allows for a larger sample and a wider pressure tube with a more simple pressure line, but one that may include a pressure reservoir and an automatic valve switching unit. This is fully described in the commercial literature of THT and THT Technical Information Sheet No 52.
Reaction Engineering
Vent sizing applications are in the area of Reaction Engineering. This is an area which the original Accelerating Rate Calorimeter was not promoted. The original system being promoted purely into the safety environment. Reaction Engineering is the area of process development and optimization. It is most often an area where reactive chemicals are in solution. The solvent is usually an organic liquid of low molecular weight and boiling point. Reactions that occur in the chemical process industry of this type, can be divided into two (or more) types of reaction. Some reactions will produce gas, others will not. Processes being carried out in the chemical industry with these reactions often have vessels where there is a burst disc which leads to a vent line. The vent line may be a pipe with bends and may terminate in a dump tank.
Process Control
In the chemical industry there is more and more emphasis in the area of process control (i.e. the design of inherently safe reactions such that the system does not require a vent or burst disc). However many engineers still need to put vents onto many vessels. Therefore they need to know what will vent (vapour, gas mixture, gas and liquid or 2-phase flow) and for calculation, they need to know what is the self-heating rates and pressure or pressure increase rates at specific temperatures. This will allow the specification of vent type and size and the burst disc venting pressure.
DIERS group
Much research and development within this area was carried out by the DIERS organization. DIERS have produced equations that can simply be applied to the differing types of reaction to allow sizing of vents. However recently there have been many modifications and some criticism of the DIERS equations used. This whole area is one of much discussion and debate and this no doubt will continue well into the future. Different groups have different opinions as to whether bench scale testing can really be carried out reliably.
THT Protocol
In the THT Accelerating Rate Calorimeter with vent size option, the sample vessel is larger than usual but not as large as that used in vent size instruments. The THT vessel takes a sample size which is large enough to be reproducible, but not dangerous. The pressure line is wider and can be automatically controlled to open and shut at pressure rates and self-heating rates and at set pressure values. There is a protocol to carry out vent sizing experiments in the Accelerating Rate Calorimeter. Firstly an experiment is carried out to determine if the system to be vented is vapour or gassy.
- Vapour: little or no gas produced by the reaction, pressure generated is from a vapour pressure rise of the solvent and or sample.
- Gassy: the reaction proceeding generates no condensable gaseous products, the pressure generated may also include the rise in vapour pressure from components.
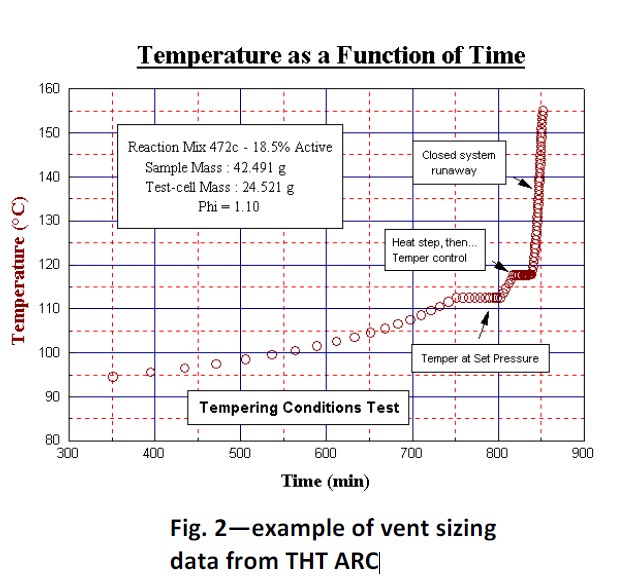
This initial distinction is necessary to determine the method of calculation of vent size. A vapour system is one that may be vented by tempering. Tempering means that if a vent is opened at a predetermined ‘set pressure’ there would be evaporation of the solvent and this evaporation will cause the reaction to slow down and stop. The solvent vaporization thus tempers the reaction. Testing at different ‘set pressures’ may be necessary to determine the appropriate ‘set pressure’.
At too high a pressure, heat generation may be too rapid to allow tempering. With this type of reaction, the self-heat rate at the ‘set pressure’ is required to allow calculation. A gassy reaction is one where opening the vent at a pressure will not lead to slow down by vaporization of solvent. The actual reaction itself will produce more heat and gas, runaway would continue. The gas produced may promote 2-phase flow – a larger vent size would be needed. Such a reaction is much harder to model and release through a vent is unlikely to be completely modelled reliably with such a bench scale instrument. If a reaction is found to be gassy, initially the pressure rate at the set pressure is required to allow DIERS calculation. The approach that has been utilized by the THT Accelerating Rate Calorimeter is to determine the type of reaction thus,
- Use as light and as large a sample contained as possible Compensate for the heat capacity of the sample contained ARC, modifying the concentration of the sample (See THT Technical Information Sheet No 24, Accelerating Rate Calorimetry at phi = 1)
- Use a wide pressure line from bomb and incorporate a gas reservoir (Fig. 1)
- Pressure the system to the relief set pressure
- Heat wait seek to the exothermic
- At the set pressure open the valve
- Wait to determine if the reaction slows down
- Close the valve; Allow to self-heat further Open valve; Wait

This double check approach will confirm if the reaction tempers. Detailed description is not given in this introductory information sheet. For further information on the low Phi cans (φ <1.1), that can be used in the Accelerating Rate Calorimeter, request specifically this information. For detailed information on use of the THT Accelerating Rate Calorimeter and Vent Sizing testing and strategy, request THT Technical Information Sheet No. 52.
하기
