Instruments to which this note applies: I-Cal Flex
Prepared by: Lars Wadsö
Target use: Stability, compatibility, sodium percarbonate
Introduction
compatibility이라는 용어는 접촉하는 서로 다른 원소가 잘 작용한다는 것을 의미하며 화학물질과 재료가 서로 접촉을 통해 사용된다는 것을 보여주기위해 화학에서 주로 사용하는 용어입니다. 반대로 화학적 비친화성은 각 물질
혹은 재료가 서로의 접촉을 통해 스스로 발생하는 것보다 빠르게 분해될 때 발생합니다. 비친화성이라는 용어는 폭발 및 화재와 같은 위험을 야기하는 경우와 분해 속도가 느린 경우 화학물질 사이에서 반응하여 발생한다. 후자의 경우, 공정은 즉각적인 위험은 발생하지 않지만 물질 또는 소재의 하나(또는 둘다)는 천천히 분해되서 원하는 특성을 잃게 됩니다.
비친화성의 일반적인 예시
- 의약품성분은 정제 제형에서 다른 구성성분과 접촉하여 분해됩니다.
- 포장 재료는 포장 제품의 열화를 촉진합니다.
일반적으로 등온열량 측정법의 호환성 테스트는 각각의 화합물과 그 화합물들이 함께 열을 만들어 비교하여 수행됩니다. 만약 두 화합물을 A와 B라고 가정한다면 그 화합물들은 A, B 그리고 화합물 A+B를 만들어 측정할 것입니다. 만약 혼합물 A+B의 열 발생량이 A의 발생량과 B의 발생량을 합친거 보다 높다면 이는 반응이 일어나는 것이고 불친화성을 의미합니다. 응용자료에 있는 경우는 B의 양은 A의 양보다 적으며 우리가 추구하는 효과는 촉매적 성질입니다. 이 경우 소량의 B는 그 자체로 측정 가능한 열을 생성하지 않는다고 가정하고 그림 1과 같이 A와 A+B 2개의 혼합물만 측정합니다.
과붕산화나트륨(SPC)는 분말 기반 세탁 제품에서 표백제로 사용되며 안정성은 가장 일반적으로 습도 및 구리와 같은 일부 금속 이온의 존재처럼 여러 요인에 영향을 미칩니다. 따라서 안정적인 제품을 위해 SPC를 건조시키고 금속이온 재료와 멀리하는 것이 중요합니다. SPC-제올라이트의 상용성에 대한 참고문헌 [1]에 나타난 바와 같이, SPC는 분말 세제 제형에 사용된 다른 물질과의 비친화성을 나타낼수 있습니다.
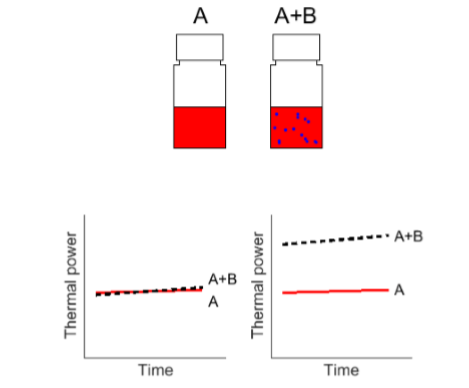
substance (B) influences another substance (A). Top:
schematic of the two test vials. Bottom: schematic examples of
when no incompatibility is detected (left) and when there is
a clear indication that B causes A to degrade (right).
Note that :
- For a comparison between the results of A, B and A+B to be valid, the results from the different measurements must be scaled in proportion to the relative mass of each compound in the final product.
- In some instances, it is not trivial to get different solid substances and materials in close contact with each other. Typically, the substances or materials are finely divided, placed in the calorimetric vial and mixed by shaking. For a more intimate contact the components can also be pressed together.
- An increased heat production from the combination of the substances/materials indicates incompatibility, but the opposite – no increase in heat production – is not necessarily proof that the materials are compatible. Reactions with a low and undetectable heat production may still cause incompatibility problems
In this application note we show the principle of calorimetric compatibility testing by investigating the compatibility between SPC and paper. This is a real world test example of relevance, since paper-based boxes are commonly used for SPC containing products. The test protocol consisted in preparing paper samples with and without copper ions, and then measuring the heat production when SPC is in contact with each of the samples, as outlined below.
Materials and methods
Two paper materials were used. Both were based on the same standard white copying machine paper:
Paper A - Treated with a weak solution of CuSO4.
Paper B - Untreated
Both papers were dried at 50% RH before the measurement.
The source of SPC use in this example was a product marked as incorporating “>30% oxygen containing bleach compound” (which typically means that it contains approximately 65% sodium percarbonate). The remaining 35% are typically mainly sodium carbonate, but also surfactants, enzymes, fragrances, etc.
Four samples were made by taking 16 g of the SPC product. Thermal powers of the product itself were measured in each of the four samples, after which pieces of paper cut into 2 mm × 2 mm pieces were added to some samples as the measurement continued. After the paper had been added, the vials were shaken manually for 45 s. The four samples were:
- No paper added.
- Paper A added (32 µgCu/gSPC).
- Paper B added.
- Paper A added (16 µgCu/gSPC).
The measurements were made at 40 °C in a Calmetrix I-Cal Flex using standard 20 mL polyethylene (PE-HD) vials. The calorimeters had previously been calibrated electrically and baselines were taken after completion of the measurements.
Results and discussion
Figure 2 shows the result normalized by mass of the SPC product. It is clearly seen that paper A (treated with copper sulfate) increases the thermal power, and that a higher increase is seen for the sample with more paper (more copper).
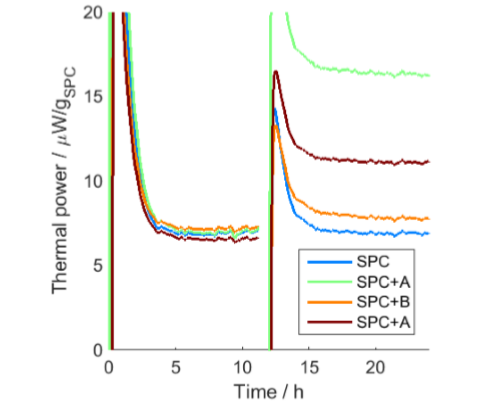
SPC with added paper materials (after 12 h).
However, Fig. 2 also shows that there is an increase in thermal power for the paper without added copper ions. This is probably an effect of the moisture content of the paper (an ordinary paper contains 5-10% absorbed moisture at 50% RH), as water is a well-known enhancer of SPC degradation [2].
Notes on proper interpretation of early parts of the calorimetric measurement
Many real world experiments present imperfect situations or results that are influenced by experimental protocols. Calmetrix application notes always show real, unaltered, experimental results, including imperfections. In this case, when the samples are initially introduced into the calorimeters, the early part of the signal seems abnormal, as it first comes from the negative side, then peaks above the final value, before settling to a quite constant value. The reason for the peak is that in this experiment, the calorimeters were not well balanced. All calorimeters had references for samples with heat capacities of about 31 J/K, which probably is significantly higher than the heat capacity of 16 g SPC. When the vials were inserted into the calorimeters, the vials had a lower temperature than the calorimeters, which translates into the initially negative values of thermal power. However, the final approach to steady-state is influenced by the samplereference balance, which in the present case results in a peak in each measurement. This peak does not represent heat production in the sample, but is an artifact caused by the nonbalanced system, and would have been absent if the calorimeters had been well balanced. Nevertheless, the stable thermal powers after the peaks are true thermal powers and can still be used to interpret the effect of copper ions on SPC degradation.
References
-
Johansson, C., P. Pekonen, and D. Forsström, The longterm stability of sodium percarbonate in presence of zeolite as measured by heat flow calorimetry. Tenside Surfactants Detergents, 2007 44 4 210-217.
-
Brundu, M. and V. Guida. Modeling the chemical decomposition of sodium carbonate peroxyhydrate. in COMSOL Conference. 2012. Milan.
