Instruments to which this note applies: Biocal 2000, I-Cal Flex/Ultra
Prepared by: Roshni Sadananda Shetty, Lars Wadsö, Federico Gómez and Björn Bergenståhl. This application note is based on the Master Thesis “Isothermal calorimetric studies of cocoa butter polymorphism” by Roshni Sadananda Shetty, Department of Food Technology and Nutrition, Lund University, Sweden 2014 [1]
Target use: Food science, crystallization of food ingredients (in this case, cocoa butter)
Introduction
초콜릿의 품질은 코코아 버터의 함량으로 결정됩니다. 코코아 버터를 원하는 결정형으로 굳게 하면 원하는 특성의 광택이 있는 단단한 초콜릿을 얻을 수 있습니다. 코코아 버터 결정화에 관한 과거 연구에서 시차주사 열량측정법(DSC) 그리고 X선 회절(XRD)와 같은 다양한 기술들이 사용되었습니다. 이 연구에서는 등온열량계와 DSC를 함께 사용하였습니다. 등온 열량계로 코코아 버터의 느린 결정화 운동을 관찰하였고 DSC로 여러 시간마다 어떠한 결정 형태가 이루어지는 지를 관찰하였습니다(DSC 결과에 대한 자세한 내용은 여기 설명하지않았습니다).
코코아 버터 즉, 초콜릿은 코코아 나무인 Theobroma cacao의 열매 알맹이로부터 얻은 지방으로 만들어집니다. 가공되지않은 코코아 콩은 공장에 도착하기 전에 발효와 건조를 시킵니다. 도착해서는 세척하고 좋은 혼합기에 넣고 섞고 분쇄 후 껍질을 제거합니다. 내부의 알맹이와 떡잎은 볶고 액체 코코아 덩어리로 간 후 높은 압력으로 코코아 버터 지방을 얻습니다. 마지막으로 남아있는 코코아 잔유물를 없애기 위해 버터를 여과합니다.
지방의 주요 성분은 트리 아실 글리세롤 (TAGs)입니다. TAGs는 글리세롤과 지방산의 에스테르이빈다. 이는 3개의 지방산이 에스테르화된 글리세롤 골격으로 구성됩니다. 코코아 버터는 주로 스테아르 산(St) 과 올레산 (O)으로 된 팔미트 산(P)의 균질 혼합물로 구성됩니다. TAG의 가장 특징적인 3가지는 코코아 버터에서 TAG의 80%를 구성하는 POP, POSt 그리고 StOSt이다.
Cocoa butter has a complex crystallization behavior and six identified polymorphs (crystal forms, Table 1). Each of these polymorphs have characteristic melting points and distinct crystal patterns associated with them. To manufacture good quality chocolate, it is essential to induce the formation and stabilization of the β2 polymorph, and this requires controlled crystallization during production. Under-tempered chocolate shows a characteristic 1 β ʹ polymorph and when the crystals transform from 1 β ʹ to 2 β ʹ, a transformation polymorph β1 occurs, which is associated with fat bloom. The metastable γ and α phases and the more stable β ʹphases can crystallize directly from the melt, but the desired β2 form can only be obtained by phase transforming the β ʹ form.
Materials and methods 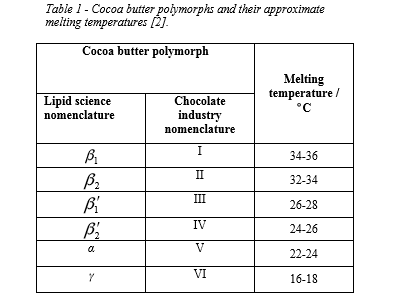
Cocoa butter
Three kilograms cocoa butter from Bühler AG was melted in an oven at 80 °C and was held at that temperature for 15 min to homogenize it and erase crystal memory [3]. The butter was then kept agitated by a Sorval Omni-Mixer (Omni International, Georgia, United States) while 10 g samples were weighed into 125 ml calorimetric plastic vials and immediately placed into 4 °C for cooling.
Seeding
Cocoa butter was recrystallized by dissolving it in pentane and rapidly cooling it to -20 °C (with a calcium chloride coolant) to create small β 1 crystals. The sample was shaken at intervals during crystallization. Once precipitated, the sample was filtered in a cold room and the crystals were dried in a fume hood to evaporate the pentane. The sample was then bottled and stored at 4 °C. The melting point of the seeds were measured by DSC to ensure they were in the required polymorphic form (β1 or β2, both these forms will induce β2 crystallization in the cocoa butter).
The calcium chloride coolant was prepared by dissolving, 77.6 g of calcium chloride (Sigma) in 150 ml of water to make a saturated solution of calcium chloride. This solution was frozen at -20 °C. On freezing, it formed a gel-like liquid which was used as a coolant for seeding.
homogenizing and subjecting it to the isothermal experiment
at 26 °C with seeding
Isothermal calorimetry
An isothermal calorimeter (BioCal 2000) was used to follow the crystallization. This instrument uses 125 ml polyethylene vials. The mass of cocoa butter used in each measurement was 10 g and the measurements were made at 26 °C. For the isothermal measurements, a homogenized sample was melted in an oven at 80 °C and was held at that temperature for 15 min. The sample was then cooled on a shaker plate at 300 rpm at 4 K/min to 29 °C. 0.1 g of the created seeds were added to the agitated samples and further cooled to 26 °C before loading the sample in the isothermal calorimeter at 26 °C.
Results and discussion
The cocoa butter was semi-solid at the time it was placed in the calorimeter, but clearly re-crystallized during the 20 h measurement, and was solid at the end of the measurement. The seeded and homogenized samples yielded reproducible results in the isothermal calorimetry measurements (measurements on non-homogenized or non-seeded samples were not reproducible and results of such measurements are not shown here). The isothermal calorimetric results showed a clear two-stage process (Fig. 2), with one process peaking after 2-3 h, while the main peak comes after about 6.5 h. These test results can be sued in a number of ways, such as to the modeling of complex crystallization behavior, using for example, the two-stage model by Foubert et al. [3].
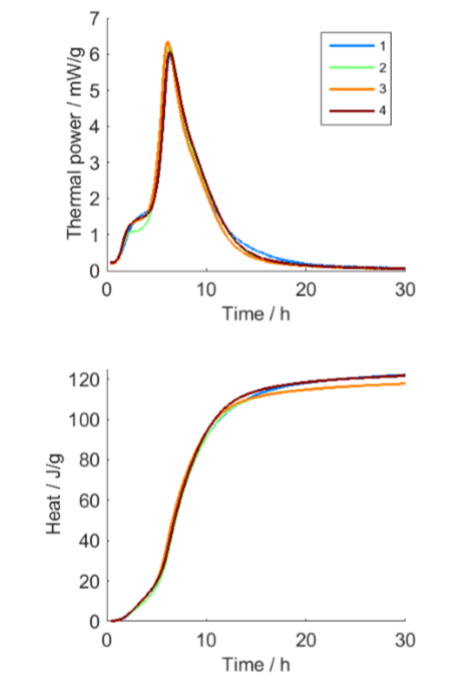
and heat (right) results for four replicate samples.
Conclusions
Isothermal calorimetry is a convenient and useful technique to follow long term crystallization processes in chocolate products. Once a suitable protocol has been established, the test procedure, sample preparation and calorimetry test itself are uncomplicated to perfrom. The measurements in this application note were done on a 24-hour timescale, but it is possible to continue measuring for longer times, given the high thermal powers from the crystallization reaction, even for small samples.
References
-
Shetty, R.S., Isothermal calorimetric studies of cocoa butter polymorphism, in Department of Food Technology and Nutrition2014, Lund University, Sweden.
-
Tannenbaum, G., Chocolate: a marvelous natural product of chemistry. J. Chem. Educ., 2004 81 8 1131-1135.
-
Foubert, I., K. Dewettinck, and P.A. Vanrolleghem, Modelling of the crystallization kinetics of fats. Trends Food Sci. Technol., 2003 14 79-92.
검색 결과 16건
- 분류 : 바이오열량계
