Safety Studies on Lithium Batteries Using the Accelerating Rate Calorimeter
Abstract
A number of Accelerating Rate Calorimeter studies have been conducted on lithium chemistry based batteries. Temperature, pressure and self- heat rate data have been recorded as a function of time within an adiabatic environment. A few examples are considered, focusing on the more common chemistries under conditions such as shock discharge and thermal decomposition at elevated temperatures. Use is made of the data as a means of quantifying associated thermal stability hazards in storage and handling scenarios.
Introduction
The Accelerating Rate Calorimeter provides a finely controlled adiabatic environment whereby heat generated by a sample is retained. It provides the user with a means of investigating samples under a thermally worst case scenario, where no heat is lost to the environment. In general the thermal dynamics of an adiabatic approximation have been shown to hold true for storage and large volume situations. This is especially the case for solids and high viscosity liquids.
Typically during a test, materials are heated in a step-wise fashion until heat generation is detected. The experimental platform is designed to be especially sensitive to heat generation, detecting self-heating from levels as low as 0.01° C/min whilst at the same time handling pressures of 300 bar (or more if necessary).

System Description
The Accelerating Rate Calorimeter system comprises the following (Fig 1):
- Calorimeter Assembly
- Safety Enclosure
- System Electronics
- PC Interface
The calorimeter assembly consists of a 25mm thick cylindrical chamber with the test cell suspended centrally within it. The chamber temperature is controlled such that it matches that of the test-cell, thus maintaining adiabaticity throughout a test. The safety enclosure protects the user from the high temperatures and pressures that typically occur during a test. It provides additional safety for the user in cases where a test-cell may rupture. The system electronics are responsible for the handling of sensor signals, driving system devices, system and logic control, and storage of test data. They also provide a limited interface for the user. The PC is the main system interface, for setting up tests and the permanent storage of data. The data analysis package ARCCal + also resides on the PC.
Note : the system described is the standard system. The EV system contains a blast box and THTs extended volume calorimeter. Contact THT for details.
Test Description
A sample is placed in a test-cell which is suspended by a connecting
tube from the lid section of the calorimeter. This is linked to a highly sensitive pressure transducer so that
pressure is monitored during a test. A temperature sensor in the form of a thermocouple is attached to the test-cell. The calorimeter lid is placed onto the calorimeter body such that the test-cell is positioned concentrically within it. The safety enclosure is closed enabling the test to proceed.
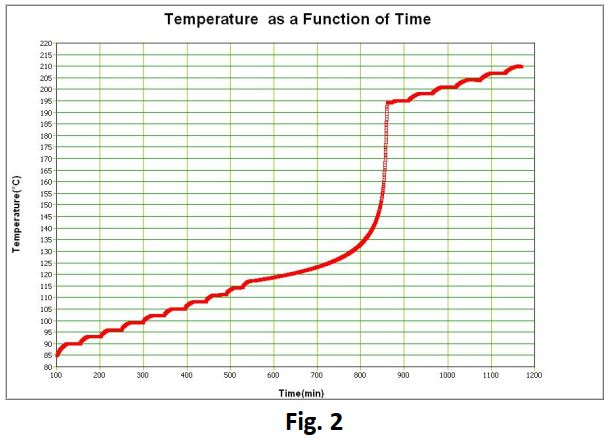
During a typical Heat-Wait-Seek test (Fig 2), the user has to specify a start and end temperature, a temperature step and a temperature rate sensitivity. Other performance and safety related parameters may have to be specified also.
The system heats the sample to the test start temperature, allows any system temperature transients to settle, and then looks for exothermic activity by comparing the temperature rate with the sensitivity. If none is observed the temperature is raised by the temperature step value requested. This procedure is repeated until the sample has been raised to the end temperature. If at any time heat generation is detected above the sensitivity threshold, the system stores temperature, temperature-rate and pressure data for downloading to the PC after the test has ended. It is important to note that adiabaticity with respect to the sample is maintained throughout the duration of the test.
Thermal Stability of Batteries
The market pressure on battery manufacturers to develop and produce batteries with ever increasing power densities has led to the use of potentially hazardous chemistries. The development in the last decade of lithium ion based batteries has raised concerns regarding manufacture, storage and customer safety. A number of leading battery manufacturers have turned to the Accelerating Rate Calorimeter as a means of quantifying the full potential of such hazards. Special test-cells for use with different battery types, offering both open and closed configurations are manufactured and supplied by Thermal Hazard Technology.
Over the last two years Thermal Hazard Technology have conducted tests under contract and also on an independent research basis. The range of lithium ion chemistries studied has included the following :
- Lithium Thionyl Chloride
- Lithium Manganese Dioxide
- Lithium Sulphur Dioxide
- Lithium Iron Disulphide
- Lithium Vanadium Pentoxide
A few of the above examples are considered in this paper.
Case Studies
(1) Thermal Stability and Decomposition in Lithium Thionyl Chloride Batteries
The thermal stability of a lithium thionyl chloride battery at elevated temperatures was investigated using the Accelerating Rate Calorimeter. The battery was of the type 2/3 A in a fully charged state with an open circuit potential of 3.5/3.65 V and a mass of 29.1g.
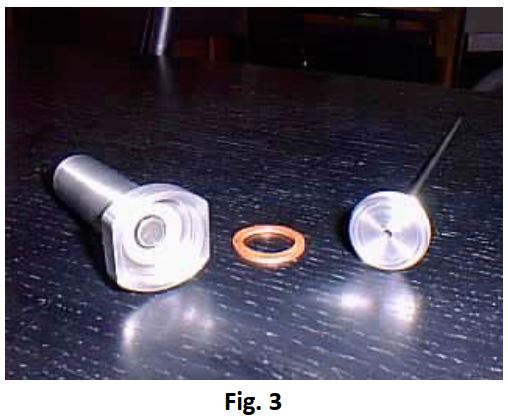
A specially constructed closed test-cell type (BAT- SS316L-2/3A) (Fig 3) made of stainless steel 316L and weighing 63.5 g was used. With a thickness of approximately 0.8mm it is capable of withstanding pressures in excess of 200 bar without leaking.
Test set-up
The battery was heated to an initial test temperature of 50°C and heated through temperature steps of 5°C. The sensitivity was set to 0.02°C/min, i.e. to half the calibrated value of 0.01°C/min. An end temperature of 200°C was selected from experience of previous similar tests. In addition, a safety cut-out pressure of 200 bar was set to prevent test-cell rupture, along with cut-outs on detection of temperature drops greater than 20°C and pressure drops of over 20 bar.
Results
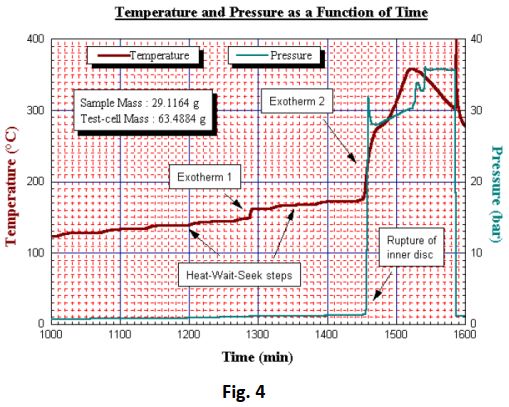
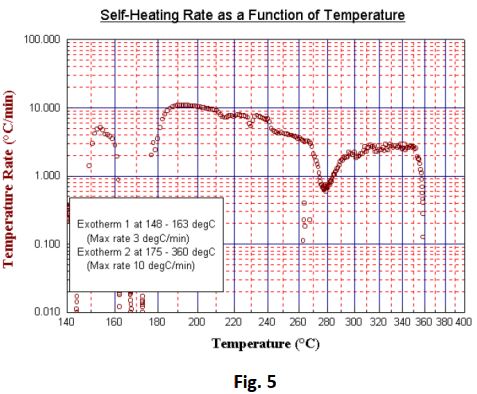
Significant exothermic activity was initially determined at 148°C giving rise to a 14°C adiabatic tempe
rature rise during which a maximum self- heating rate of 5.1°C/min was noted (Fig 4 and Fig 5 overleaf).
Following two more temperature steps a second and much more significant exotherm was detected. This was picked up at 172°C and reached heating rates of 10.8°C/min over prolonged periods of time. The temperature rose to 358°C despite the test being terminated at 200°C and cooling air being applied. Post-test examination of the test cell showed that the decomposition by-products had corroded a hole of approximately 10 mm in the side of the test- cell. It therefore appears that the maximum observed pressure of 40 bar was sustained during leakage and the pressure rate may well have exceeded the maximum recorded value of 11 bar/ min. Following two more temperature steps a second and much more significant exotherm was detected. This was picked up at 172°C and reached heating rates of 10.8°C/min over prolonged periods of time. The temperature rose to 358°C despite the test being terminated at 200°C and cooling air being applied.
Conclusions
The flat self-heat rate peak which seems to have occurred as a result of the mass loss and probable heat loss through boiling of volatiles from the sample prevents a detailed analysis of the data from being performed. Despite such restrictions the Accelerating Rate Calorimeter data may be used to identify quantitatively storage temperature limits. In this case, rule of thumb suggests that temperatures within a warehouse storing the investigated lithium thionyl chloride batteries should not exceed 100°C such as may be encountered in hot climes and would certainly result in a fire scenario. In addition the qualitative data provides evidence as to the possible consequences that may result if such advice is ignored. Apart from a pressure rise at considerable rates, the corrosive effects of the generated gases have also established unequivocally the need for additional environmental considerations where storage is concerned.
Stock Discharge Studies of Lithium Iron Disulphide Batteries
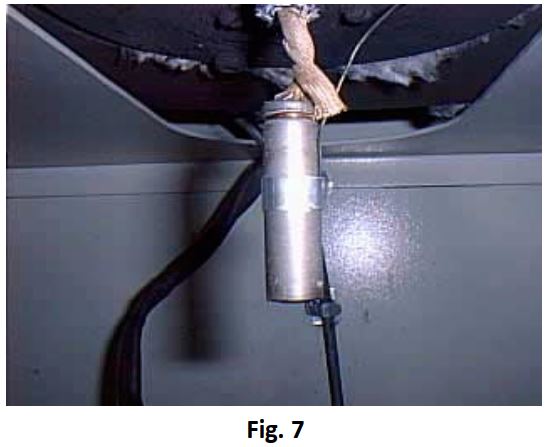
The thermal behaviour under adiabatic conditions of an AA size 1.5V lithium iron disulphide battery undergoing shock
discharge was investigated. Unlike most tests in the Accelerating Rate Calorimeter, the aim of the investigation was to consider the fairly likely scenario of shorting a battery in a well insulated pocket of a customer, such as when placing it amongst loose coins. The battery was therefore maintained at atmospheric pressure during the entire test by not using a test cell. Instead the discharge leads and the thermocouple where used to hold the battery in position (Fig 7). A small aluminium band ensured good contact of the thermocouple with the battery. The battery had a mass of 20.0 g and was fitted internally with a thermal cut-out safety device.
Test set-up
A test start temperature of 37.5°C was used in an attempt at simulating a customer’s pocket temperature. An end temperature of 250°C was selected, and temperature steps of 5°C with a sensitivity of 0.02°C/min. The battery was allowed to proceed through its initial Heat-Wait-Seek cycle. During the exotherm seeking period the battery was shorted so that it would shock discharge. A sudden exotherm resulted, with adiabaticity being maintained and data being collected.
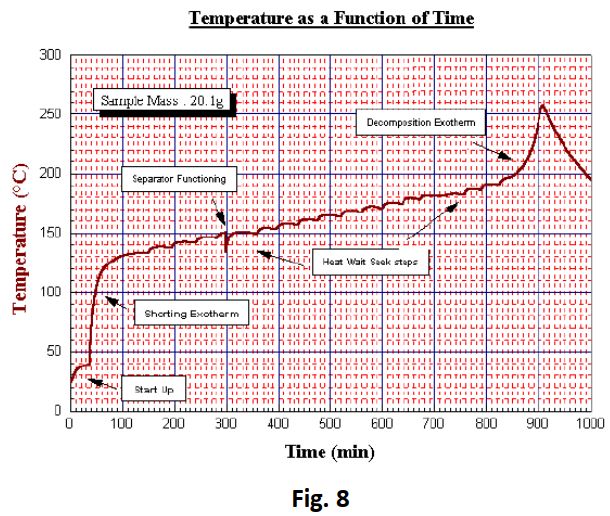
Results
The shock discharge produced a very sudden but prolonged temperature rise (Fig 8 and Fig 9). The temperature rose by 95°C to over 133°C, during which the battery rupture disk appeared to remain intact. From the plot it is possible to establish from a sudden drop in temperature at 150°C that the rupture disk vented at this point. Subsequent heating steps resulted in a second exotherm being detected at 197°C. This was most probably the result of a thermal decomposition. The test exceeded the end temperature of 250°C and was therefore terminated prematurely before the natural end of the exothermic process. No pressure data was collected since the battery had been open to atmosphere.
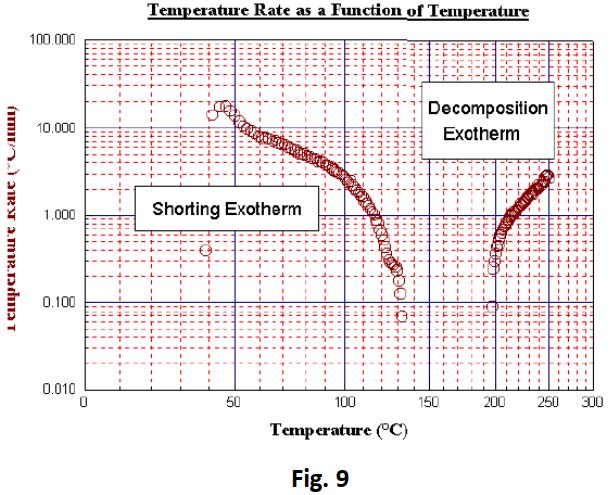
Conclusions
A 95°C rise in temperature is of considerable concern,
particularly since it was apparently protected by a thermal cut-out. From the data alone it is evident that a customer may experience temperatures in the region of 120°C to 130°C (possibly in a coat pocket!) if certain conditions are encountered accidentally. The possibility of shock discharge during storage raises the question of risk to warehousing. If the rule of thumb approach is used to estimate a safe temperature for the thermal decomposition reaction, this would be around 150°C. This value applies to a fully discharged battery. It is very likely that in a fully charged state this temperature limit may fall to below the 133°C maximum temperature which was observed during the discharging stage of the experiment. Hence batteries discharging adjacent to ones that maintain their full charge may lead to unforeseen complications.
Thermal Decomposition Study of Lithium Manganese Dioxide Batteries

The thermal stability at elevated temperatures of a 2/3 A size 3V lithium
manganese dioxide battery was studied. The test was conducted in a closed test cell type BATSS316L- 2/3A. Experimental conditions were identical to those used in the first case study.
Results
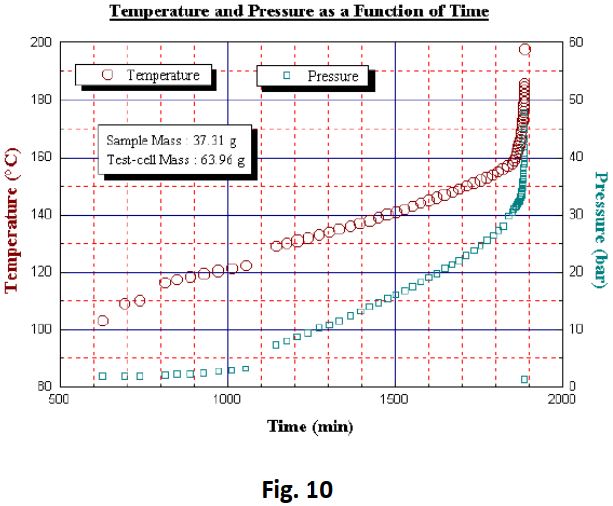
A very slow exotherm was detected from temperatures as low as 102°C (Fig 10 and Fig 11). The instrument was detecting close to its calibrated sensitivity limit and consequently introduced a couple of 5°C heat steps. The third and final exotherm was detected at 128°C, which although characteristically slow at first led to a very sharp rise in temperature and pressure at 157°C. A test-cell rupture occurred splitting the test-cell extremely violently. A maximum self- heating rate of 292°C/min was observed and just prior to detonation a pressure rate of 12 bar/min was recorded along with a maximum pressure of 48 bar. A maximum temperature of 293°C was also recorded, despite test termination at 200°C with the application of cooling air. A temperature rise greater than 191°C was observed. The remains of the test-cell are shown in Fig 12, yet in spite of such a violent event, the calorimeter remained unscathed although slightly dirty. The test cell in place before the test is shown in Fig 13.

Analysis
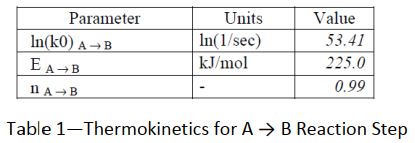
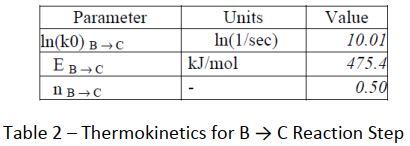
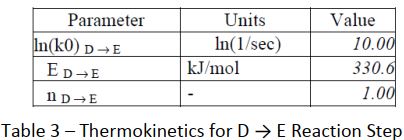
This result shows a more typical picture of thermal decomposition in lithium batteries. Use is made of the CISP ChemInform Ltd software suite to attempt an analysis of the data. The packages ADPro and Fork were used. ADPro provides an alternative platform to ARCCal+ for handling the Accelerating Rate Calorimeter data, whilst Fork enables the user to determine parameters for complex thermokinetic models. A formal analytical approach is used with an nth- order reaction scheme. The possible reaction routes were defined thus : A → B → C and D → E
Where the Arrhenius based relationship..
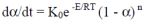
....is assumed to hold for each reaction stage.
A statistical approach using a tensor method was used to solve the thermo kinetics in this instance. The following parameters were determined (Tables 1 to 3) :
 |
 |
 |
A comparison between decomposition behaviour resulting from the derived thermokinetic model and the test data may be seen in Fig 14. Improvements to the fit are possible if the reaction schemes and models are refined further to allow for diffusion effects etc. However the user must have a good understanding of the battery which is under investigation, so that appropriate assumptions can be made.
Conclusion
A typical battery decomposition reaction for a fully charged cell was observed. The data was handled using the ChemInform Ltd Thermal Safety Software to generate appropriate thermokinetic data. Use of this may be made in estimating safety criteria during manufacture as well as storage and handling. The re-engineered instrument was re-designed to maintain the high standards set by the original system developed in the late 1970s whilst providing significant improvements in versatility in the form of options and alternative testing procedures. Quantifying battery safety has become an application area in which an ever increasing interest has been shown. Apart from the specialised test-cells that are available are also the GSU and BSU options directed at the needs of the battery manufacturing industry.
References :
ARC Technical Information Sheet No. 065, Thermal Hazard Technology, UK
D.I. Townsend, J.C. Tou, Thermal Hazard Evaluation by An Accelerating Rate Calorimeter, Thermochimica Acta, 37 (1980) 1-30 W.B. Ebner (Honeywell Power Sources Center, Horsham, Pennsylvania 19044, USA),
D.W. Ernst (Electrochemistry Branch, Naval Surface Weapons Center, White Oak, Maryland 20910, USA), Safety Studies of The Li/SO 2 Syatem Using Accelerating Rate Calorimetry, 1983
K Hasegawa, S Okawa, Y Arakawa, Safety Study of Electrolyte Solutions for Lithium Batteries using Accelerating Rate Calorimetry,
Yokkaichi Research Center, Mitsubishi Petrochemical Co. Ltd., 1 Toho-cho, Yokkaichi City, Mie 510, Japan, 1992
상세한 사항은 아래 첨부된 자료를 참고 부탁 드립니다.
하기
